Vertebral Arteries and Cervical Rotation: Modeling and Magnetic Resonance Angiography Studies
Haynes MJ, Cala LA, Melsom A, Mastaglia FL, Milne N, McGeachie JK
The Department of Anatomy and Human Biology,
The University of Western Australia, Australia
OBJECTIVE: To determine whether lumen narrowing in vertebral arteries during atlanto-axial rotation is due to stretch or localized compression.
DESIGN AND SETTING: Experiments with models were made in a private chiropractic clinic, whereas studies of cadaveric specimens were performed in an anatomy laboratory. Doppler ultrasound and magnetic resonance angiography (MRA) studies were carried out in the radiology department of a public hospital.
PATIENTS: Eight patients had their vertebral arteries examined by use of a Doppler velocimeter and MRA. Main Outcome Measure: Stenosis of the vertebral arteries caused by stretch, localized compression, or kinking.
RESULTS: All 16 vertebral arteries from the 8 patients displayed no changes in their lumen dimensions with full cervical rotation, although curves in each of the arteries did change. The model and cadaveric vertebral arteries demonstrated localized compression or kinking of the vessel wall with atlanto-axial rotation contralaterally but revealed no evidence of major contribution of stretching to stenosis.
CONCLUSIONS: The lumen of vertebral arteries is usually unaffected by atlanto-axial rotation. In cases where there is stenosis, this is mainly due to localized compression or kinking. These findings are relevant to premanipulative screening of vertebral arteries with Doppler ultrasound scanning.
From the Full-Text Article:
Discussion
The results indicate that stretch of 65% would be required to reduce the cross-sectional area of the vertebral artery (without added intraluminal pressure) by 56%, which is approximately what would be required to cause changes in Doppler signals. [14] Previous duplex scanning studies indicated that asymmetry in diameter between paired vertebral arteries of 1.5 mm (ie, a difference in cross-sectional area of approximately 56%) was required to cause substantially lower blood flow velocity in the smaller artery. [9] Theoretically, the most stretch that could have been placed on the model artery during atlanto-axial rotation (ie, with a completely straight section of vertebral artery and no concomitant C1/C2 lateral flexion) in this study was 42%. Therefore stenosis sufficient to cause changes in Doppler signals from vertebral arteries during cervical rotation is unlikely to be due to stretch being applied to the artery, as suggested by Stevens [10] and Refshauge. [11]
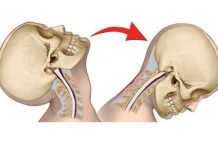
The mercury-filled artery contained within the model cervical spine demonstrated that the artery was kinked, while the cadaveric specimen showed that it was compressed during contralateral cervical rotation, as the vessel left the C2 transverse foramen. Both kinking and compression of the vertebral artery as it left the C2 transverse foramen during rotation was also demonstrated in the cadaveric studies of Selecki. [15] Indentation as seen in the model artery from the third and fourth spinal nerves was not observed in any of the present MRA studies, therefore this finding was probably due to artifact perhaps caused by the exaggerated size of the spinal nerves in the model. Wiseman et al observed that from a total of 84 vertebral arteries examined using arteriography 95% of the arteries bent laterally through the C2 transverse foramen usually at an angle of approximately 90°, whereas the remainder showed little or no deviation from their vertical course. [16] Our mercury-filled model had only a moderate bend in the tubing whereas the cadaveric specimen had a well-developed bend at the C2 level. (When handling sections of model artery, whether intramural pressure was applied or not, the straight models were more susceptible to kinking when bent than the curved model). It seems likely then that curvature in the vertebral artery decreases the risk of kinking of the vessel during cervical rotation. Dumas et al8 in their MRA study of vertebral arteries found that 14 right-sided vertebral arteries that were scanned displayed osteovascular impingement within the C2 transverse foramen as the vessel left the axis foramen. [8] One factor that Dumas et al8 had found that increased the likelihood of compression was poorly developed atlanto-axial curves of the vertebral artery. In this study the atlanto-axial curves also would have reduced the amount of traction on the model artery to less than 1/3 compared with artery model without any curves. Hence, the overlengthening of this section of the artery may be a protective feature, as proposed by Braakman and Penning. [17]
An incidental finding that was consistent in this study was of some straightening of the arterial curve in the transverse foramen of the axis during contralateral cervical rotation. This suggests that the vertebral artery may not be firmly bound by attachments of its adventitial fibers to the periosteum of the C2 vertebra at this site. Some mobility of the artery combined with its curve here would allow a large degree of lateral flexion to occur between C2 and C3, which accompanies cervical rotation, without placing stretch on the artery at this level.
In vivo studies by Mimura et al, [18] who used stereo photogrametric radiography, and Dumas et al,8 who used 3D computed tomography scanning, demonstrated that with atlanto-axial rotation there frequently was concomitant lateral flexion between C1 and C2 on the contralateral side. The model of the upper cervical spine that we used had anatomic structures that included alar ligaments and articular cartilage on the adjoining C1/C2 facets. These structures caused an automatic lateral flexion between C1 and C2 contralaterally during atlanto-axial rotation. The wire model indicated that the amount of traction applied to the artery would have been reduced to almost half just by virtue of the C1/C2 lateral flexion. Hence, 2 important factors that may reduce elongation of the artery during rotation are the curves in the atlanto-axial segment of the artery and concomitant C1/C2 lateral flexion. According to our model when these 2 factors are combined they can completely cancel out stretch that would have otherwise been applied during separation of the atlas and axis transverse processes in the horizontal plane.
The cadaveric specimen had demonstrated localized compression of the artery at C2 with contralateral cervical rotation. This specimen had displayed C1/C2 lateral flexion but it was not pronounced, and no additional intramural pressure had been applied to the artery. These factors may have been responsible for the amount of compression observed at C2. Selecki [15] had varied intraluminal pressures in cadaveric specimens by pumping water through the vertebral arteries with different pressures as observed by the height of the water jet above the artery. Although the pressure of the water in the artery was not provided by Selecki, [15] the effect of cervical rotation on the flow was found to be influenced by the intramural pressures; a higher pressure resulted in less stenosis. This feature was also observed in this study with pressures ranging from an equivalent low diastole (ie, 40 mm Hg) to high systole (ie, 180 mm Hg).
When stenosis of the vertebral artery occurred in this model and cadaveric studies, it did so toward the end range of atlanto-axial rotation, which was approximately 45°. Dumas et al8 observed that osteovascular impingement of the vertebral artery was more likely to occur when atlanto-axial rotation exceeded 35°. Previous duplex ultrasound scanning [9] and Doppler velocimeter studies [4] have indicated that when major changes in blood flow velocities occurred during cervical rotation, they did so close to the limit of rotation. Therefore the amount of atlanto-axial rotation is likely to be an important factor in determining the extent of stenosis of the artery.
In this MRA study no sign of stenosis was observed in the 16 arteries that were scanned, although all arteries displayed changes in the curves of the atlanto-axial segment. It is possible that the atlanto-axial curves and the concomitant lateral flexion of the C1/C2 segment (on the contralateral side to rotation) may have been why the arteries were not affected by rotation. In the study by Haynes and Milne [8] the duplex scanner could only obtain images of the caudal end of the atlanto-axial segment and only assess the curvature in the coronal plane. The MRA scans were able to clearly reveal the whole segment and present it fully rotated about a vertical axis, thereby providing a multitude of sagittal, coronal and oblique views. This facility enables examination of all aspects of the vertebral artery anatomy. The curves of the C1/C2 segment appear to occur in both the sagittal and coronal planes, so that the artery takes on a helical shape.
Duplex scanning studies by Haynes and Milne [9] showed localized compression of 2 vertebral arteries during cervical rotation that occurred as the arteries left C2, and the compression resulted in a jetting effect of the blood flow at this level. Five other vertebral arteries in the duplex scanning study showed major decreases in blood flow velocities during cervical rotation, but the scanning revealed no mechanism that was responsible (no signs of stenosis were seen). The MRA studies of Dumas et al [8] indicate that vertebral artery compression can occur within the C2 transverse foramen and if so, may explain why the duplex scanning was unable to detect the stenosis. Bone surrounding the foramen may have blocked the ultrasound beam, thereby making it impossible to obtain images of the artery and, hence, observe any compression of the vessel. These modeling studies also indicate that compression of the artery can occur within the transverse foramen. Whether duplex scanning can obtain images of the vertebral artery within the C2 transverse foramen may depend on the angle of the ultrasound beam and the geometry of the transverse foramen.
A number of duplex scanning studies have examined the blood flow velocities of vertebral arteries during cervical rotation [9, 11] or during combined cervical rotation with extension. [19-20] A weakness in these studies is that they had failed to make repeat measurements for each artery for each neck position or neglected to average the velocities, [11, 19, 20] or had not made an adequate number of repetitions. [9] This could have resulted in random errors of measurement [21] sufficiently large enough to cause inaccurate data related to small changes in the blood velocities. For the same reasons, the blood flow studies by Licht et al, [22] who used duplex scanning, could have also been prone to random errors when measuring the diameters and blood velocities of the vertebral arteries to determine the flow rates. Results of an earlier study by Licht et al [23] indicated that determining vertebral artery blood flow with a duplex scanner was reasonably accurate for measurements made in a group study, but not for single measurements. Large differences in blood velocities or arterial diameters would have been less affected by the relatively small inaccuracies of the duplex scanner.
More accurate data seem to have been obtained in the recent study by Licht et al, [24] who used ultrasonic transit time flowmetry to measure blood flow in the vertebral artery of pigs. Measurements were made before and then every 20 seconds after the administration of a predominantly lateral flexion type of high-velocity cervical manipulation; the measurements were continued until the flow volumes had returned to the baseline values. The results indicated that there was a moderate increase in the volume flow that occurred briefly for the first 20 to 40 seconds after the manipulation.
Licht et al [24] offered no explanation for these results. Perhaps a somatovisceral reflex, elicited by the cervical manipulations, was responsible for the temporary increase in vertebral artery blood flows. Another possible explanation is that the increase in blood flow was a response to a transient stenosis induced by the manipulation. There does not appear to be data provided for the blood flow during the time of the manipulation and then leading up to the next measurement [20] seconds later. [24] It is possible that there were initial sudden decreases in the volume flows during the manipulations.
Using duplex scanning, Haynes [5] observed an unusual response of the vertebral arteries of 1 woman subject as she moved her head back to the neutral position from full cervical rotation. There was an immediate, but transient, marked increase in the blood velocities, above the baseline values (ie, when the neck was in the neutral position) of the contralateral vertebral artery. Both of the woman’s vertebral arteries had also displayed dramatic decreases in the blood velocities toward the end range of contralateral cervical rotation. No other vertebral arteries from a sample of 39 had shown such a major initial drop in blood velocities followed by a large transient increase above the baseline. [5] This finding was interpreted as being a hyperemic response, in which a temporary ischemia can trigger vasodilation in the vascular bed resulting in decreased vascular resistance and, hence, increased blood flow. This woman participant had no vertebrobasilar insufficiency signs or symptoms during the scanning, which suggests that the ischemia was very mild.
The increased flows in the pig vertebral arteries, as observed by Licht et al, [24] might have been hyperemic responses to transient but marked stenoses of the vertebral arteries caused by the neck manipulations. If stenosis had occurred, it is likely that the main contribution to it would have been from localized compression of the artery. One reason Licht et al [24] gave for choosing pigs to be the experimental models was because the vertebral arteries of pigs, like those from human beings, pass through transverse foramina. The results of these studies suggest that a tubing model of the vertebral artery with an added intramural pressure is affected more by localized compression than stretch when tractioned over the rim of a transverse foramen. Probably the same applies to the vertebral arteries of pigs. However, further experimentation will be required to determine the biomechanical effects of cervical manipulation on vertebral arteries.
Licht et al [24] had found no changes in the blood flow of the vertebral arteries from pigs that had their heads moved into full rotation and extension. There could have been minor stenoses of the vertebral arteries, due to this positioning of the pigs’ necks, some distance away from the ultrasound transducer that had not affected the overall flow rate. Such minor stenosis could be indicative of mechanical stress to the arterial wall. The studies of Licht et al [24] indicate that vertebral artery blood flow in pigs is usually unaffected by cervical rotation and extension, but there is still the possibility that these movements could profoundly affect some pig vertebral arteries, in a similar way to human vertebral arteries. The sample size (n = 8 pigs) of their study was quite small and may not have been large enough to have included such unusual presentations.
Doppler velocimeter studies by Haynes [4] indicated that major changes in blood velocities of human vertebral arteries resulting from cervical rotation occur in approximately 5% of arteries. From all 16 vertebral arteries in the present study no sign of stenosis was observed, which provides further evidence that these vessels are generally not affected by cervical rotation. However, in patients with a history of transient ischemic attacks in the vertebro-basilar territory, stenosis caused by cervical rotation was seen in 33% of patients from the Doppler velocimeter study of Arnetoli et al, [25] and 56% of patients in the MRA study of Weintraub and Khoury. [7] The over representation of patients with transient ischemic attacks compared with matched control subjects from both these studies indicated positional stenosis of vertebral arteries constituted an independent risk factor for stroke. The exact mechanism responsible for this increased risk of stroke is not known.
The most common manifestation of vertebral artery injury causing stroke that has been associated with cervical manipulation is arterial dissection. [2] One mechanism that has been proposed is that manipulation may cause the artery to be overstretched, thereby causing intimal disruption followed by dissection. [26] There is evidence that a major factor in aneurysm formation of arteries is a defect in the elastic skeleton of the arterial wall. [27] Merel et al [28] obtained elastin fibers from intracranial vertebral arteries and was able to stretch the fibers to twice their length without any noticeable damage. It seems highly improbable that manipulation would cause stretch of vertebral arteries along the whole C1/C2 segment exceeding 100%, when maximal stretch with full rotation is likely to be less than 50%. However, at the point of compression of the artery, the deformation of the wall would result in localized stretch compounded by the pulsation of the wall with each cardiac cycle and also the stretch applied along the C1/C2 segment. With stenosis very high, blood flow velocities may also occur at the site of compression. A combination of focal tension of fairly high magnitude in the wall and blood striking the wall with considerable force because of focal constriction may cause microtrauma sufficient to weaken the wall. This may be especially so if the forces that are applied are strong or repeated.
Fibromuscular hyperplasia is an arteriopathy that is associated with arterial dissection, and it has a predilection for medium-sized elastic arteries, such as the vertebral artery. [29] In the vertebral artery, fibromuscular hyperplasia has a preference for the atlanto-axial segment, and Vega Molina et al [30] proposed that this may be due to the mechanical stresses to which the C1/C2 section of the artery may be prone. Perhaps then a genetic or congenital defect of the arterial wall could be made worse by repeated compression or stretch.
Johnson et al [31] found that longitudinal tractioning of strip specimens of cervical segments of human vertebral arteries, obtained at autopsy, caused rupture of the arterial wall with an average of 33% stretch, and in some cases as low as 12%. Johnson et al31 did acknowledge that their results may not represent whole segments of vertebral arteries in vivo, but made the point that arterial dissections probably could result from less tension than that required to cause complete rupture. These studies by Johnson et al [31] suggest the possibility that cervical manipulation could cause overstretching of a healthy vertebral artery, but one that is affected by faulty biomechanics, leading to intimal disruption.
In summary, positional stenosis of a vertebral artery is probably indicative of localized compression of the vessel, which may be injurious to the arterial wall if it is weakened by an arteriopathy. A finding of positional stenosis, and hence compression of the vertebral artery may provide an early warning of major impending stretch that could occur with further rotation. Such tractioning would probably increase the mechanical stress to the artery and increase the risk of intimal tearing. There is evidence that simple hand-held Doppler velocimeter examination can detect major stenosis of vertebral arteries during cervical rotation,5 and the present studies suggest that by doing so this may provide indirect evidence of stress to the arterial wall. If so, Doppler ultrasound scanning may be useful in the premanipulative screening of patients.
Conclusion
The results of this study do not support the hypothesis that lumen narrowing of vertebral arteries generally occurs during contralateral cervical rotation and that stenosis when it occurs is due to stretching of the vessel. It seems that when stenosis happens it is mainly due to localized compression, usually at the level of the C2 transverse foramen. Factors that may influence the degree of stenosis are the amount of atlanto-axial rotation and the concomitant C1/C2 contralateral lateral flexion, the intraluminal pressure and how well developed the curves are of the atlanto-axial segment.