Spontaneous Cervical Artery Dissections and Implications for Homocysteine
Anthony L. Rosner, PhD
Foundation for Chiropractic Education and Research
1330 Beacon Street, Suite 315
Brookline, MA 02446-3202 USA
Introduction
Over the past 5 to 10 years, the issue of cerebrovascular accidents (CVAs) and spinal manipulation has become a debate of ever-increasing intensity. A copious number of studies have investigated spinal manipulation as a putatative causative factor of CVAs [1-5]; however, a common theme among these is the failure to consider that the majority of vertebrobasilar accidents (VBAs) may be spontaneous, cumulative, or caused by factors other than spinal manipulation. The problem is not served by the sometimes hysterical reactions apparent in the media over the past 2 years in reaction to the flawed investigations. [6-11] In light of these recent reports, the entire phenomenon of spontaneous cervical artery dissections should be revisited to put this matter into a better perspective.
Dissection mechanism
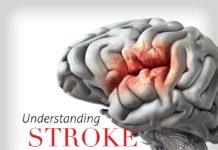
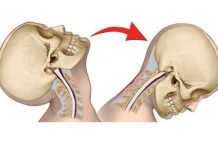
The mechanism of CVAs involves the vertebral and carotid arteries of the upper cervical spine. Following cervical manipulations in particular, the most common site of injury to the vertebral arterial system appears to be in the vicinity of the atlanto-occipital joint, where the vertical artery bends into a horizontal configuration, as shown in Figure 1. By a large majority, most postmanipulative strokes and stroke-like symptoms occur in the segment of the vertebral artery bounded by the transverse foramen of the C2 vertebra and the foramen magnum. [12] Interestingly, it is precisely in the C1-2 region in which rotational maneuvers have been suggested to produce a slight elevation in the incident rate of CVAs. [13] Trauma to the arterial wall occurs, producing either vasospasm or damage to the arterial wall itself, and subsequently leads to brain ischemia.
As described by Terrett, [14] arterial traumatic mechanisms may be classified by 1 of 6 categories:
(1) subintimal hematoma,
(2) intimal tear,
(3) intimal tear with embolic formation,
(4) vessel wall dissection with subintimal hematoma (dissecting aneurysm),
(5) vessel wall dissection with pseudoaneurysm formation, and
(6) perivascular bleeding (false saccular aneurysm).
Although one might suspect altered vertebral artery hemodynamics and reduced basilar perfusion to be consequences of diagnostic testing and/or spinal manipulation, it is primarily the vertebral arterial dissections (VADs) which have been implicated in the majority of stroke or stroke-like incidents [12] to be directly attributable to spinal manipulation. VADs, therefore, will form the basis of the ensuing discussion.
Spontaneous VAD rates
As shown in Table 1, the annual incidence of spontaneous VADs in hospital settings has been estimated to occur at the rate of 1 to 1.5 per 100,000 patients. [15] The corresponding VAD incidence rate in community settings has been reported to be twice as high. [16, 17] Using an estimated value of 10 from the literature to represent an average number of manipulations per patient per episode, [23] it becomes apparent that the proposed exposure rate for CVAs attributed to spinal manipulation is equivalent to the spontaneous rates for cervical arterial dissections as reported. [15-17] If the threat of stroke or stroke-like symptoms is to be properly assessed, therefore, at least half our attention needs to be directed toward the spontaneous events instead of primarily or solely on spinal manipulation.
Table 1. % Rates of stroke compared with incidence of arterial dissections
Attributed cause Rate (per million)
Spontaneous, hospital-based [15] 10-15
Spontaneous, community-based [16, 17] 25-30
Cervical manipulation [18] 25
Cervical manipulation [19] 10-20
Cervical manipulation [20] 0
Cervical manipulation [21] 6.4
Cervical manipulation [22] 1.7
Corrected to represent the average incidence per patient assuming the average number of manipulations per patient to equal 10, as reported in the literature. [23]
Genetic factors and elevated homocysteine
A consortium of investigators from northern Italy have recently demonstrated that a genetic defect in humans (C677T MTHFR, a thermolabile variant of the enzyme tetrahydrofolate reductase with half the normal activity) is associated with elevated levels of the amino acid homocysteine. [24] According to Figure 2, A and B, this is shown
(1) by the failure of the reductase enzyme to generate sufficient quantities of N5-methyltetrahydrofolate (Fig 2, A), resulting in
(2) the inability to convert sufficient quantities of homocysteine to methionine (Fig 2, B).
This is due to the fact that there are inadequate amounts of the methyl donor cofactor N5-methyltetrahydrofolate to catalyze the reaction, with the result that the precursor to methionine (homocysteine) accumulates intracellularly. [25]
In comparing 3 groups of about 30 patients each, Pezzini et al [24] indicated that spontaneous cervical artery dissections (sCADs) are represented in the pool of patients whose homocysteine levels exceed 12 µmol/L—more than 3 times as much as asymptomatic patients and more than twice as much as patients who have undergone ischemic strokes without arterial dissection. Direct correlations of elevated plasma homocysteine levels with the occurrence of sCAD were also demonstrated, [24] a finding that is echoed elsewhere by the findings that
(1) cervical artery dissection (CAD) patients had average homocysteine levels of 17.9 µmol/L, while asymptomatic patients reported 6.0 µmol/L26; and
(2) homocysteine levels exceeding 10.2 µmol/L are associated with a doubling of vascular risk. [27]
What is the clinical significance of elevated homocysteine levels? For years, homocysteine has been implicated as a key component of atherosclerosis and cardiovascular diseases, [27-35] but Pezzini et al [24] and other investigations suggest a more direct role. A significant number of clues all point toward the disruption of the structure of collagen and elastin in the arterial wall:
-
In the majority of skin biopsies taken from patients with cervical arterial dissections, irregular collagen fibrils and elastic fiber fragmentations have been found. [36]
-
Homocysteine activates metalloproteinases [36] and serine elastases, [37] directly or indirectly leading to the decrease in vitro of the elastin content of the arterial wall. The opening and/or enlargement of fenestrae in the medial elastic laminae would be expected to lead to the premature fragmentation of the arterial elastic fibers and degradation of the extracellular matrix. [36, 37]
-
Homocysteine has been shown to block aldehydic groups in elastin, inhibiting the cross-linking needed to stabilize elastin. [38]
-
The cross-linking of collagen may also be impaired by homocysteine. [39]
All of these observations would be enhanced by elevated levels of homocysteine and suggest that the resulting potential defects of the extracellular matrix of the vessel wall may play a role in the pathogenesis of arterial dissection.
The Pezzini et al [24] observations concerning a defective tetrahydrofolate reductase may extend to a second mechanism by which collagen structure is disrupted. As shown in Figure 2, A, the aberrant enzyme leads to an accumulation of the precursor N5,N10-methylenetetrahydrofolate. It is conceivable from the biochemical pathway shown in Figure 3 that this entity may then favor the catabolic conversion of glycine into serine. [25] By coincidence, glycine, as the smallest existing amino acid, turns out to be an essential component in the repeating tripeptide sequence which constitutes collagen. As shown by Figure 4, virtually all the hairpin turns in the core of the helical structure of collagen are glycine residues [25]; any lack or substitution thereof would be expected to impart significant kinks and other aberrations to the collagen structure, rendering it more susceptible to spontaneous degradation.
Other links between elevated homocysteine and CAD
Links between increased plasma levels of homocysteine and vascular disease were proposed as early as in 1969. [28] After a continuous 3-month infusion of homocysteine in baboons, vascular injury and thrombosis could be induced with patchy endothelial desquamation, observed on up to 10% of the aortic surface. [33] In subjects with hyperhomocysteinemia, an impaired reaction of endothelium-dependent and flow-mediated dilation could be observed. [34] Finally, in cell culture experiments, the addition of homocysteine into the cell medium induced the detachment of cells from the endothelial cell monolayer. [40] The common denominator pertaining to risks associated with spinal manipulation is arterial wall fragility, rather than stenosis and the other cardiovascular risks which have been associated with elevated homocysteine. [28-32]