Jennifer Molson was 21, juggling a day job and night school to pursue her dream of becoming a cop, when she was diagnosed with multiple sclerosis. She woke up one morning with pins and needles in her hand, and within a week she couldn’t move her left arm.
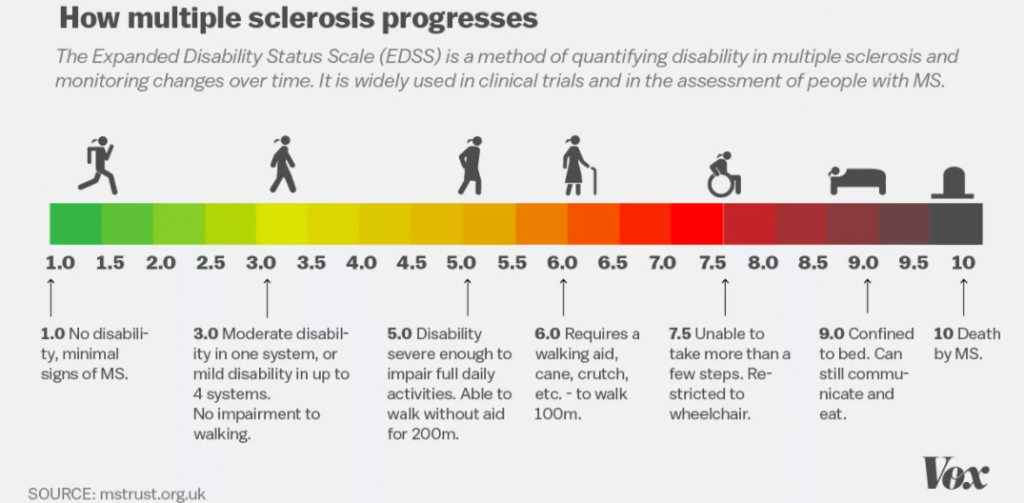
By 2001, five years later, she was living in the Ottawa Hospital under 24-hour care, getting around using a cane, walker, or wheelchair. When she was discharged on weekends, to spend time with her then-boyfriend Aaron, she had to rely on him for her every need. He’d cut her food into bite-size pieces, and bathe and dress her. When she lost control of her bladder or bowel, he’d help her go to the bathroom.
 “I had no feeling from the chest down,” Molson says. “I could touch something boiling on the stove and burn myself. I could touch fabric without knowing whether it’s sandpaper.” For patients like Molson, with a severe form of MS and no response to the available medications, there’s little hope.
“I had no feeling from the chest down,” Molson says. “I could touch something boiling on the stove and burn myself. I could touch fabric without knowing whether it’s sandpaper.” For patients like Molson, with a severe form of MS and no response to the available medications, there’s little hope.
Two years later, her life had taken an abrupt turn. “I walked down the aisle and danced at my wedding, something I had always dreamed of doing,” she said of her marriage to Aaron in 2003.
Now, 15 years later, Molson is still skiing and kayaking on the weekends. She works as a research assistant at Ottawa Hospital.
What happened is something even esteemed medical specialists are venturing to call a “miracle”: The particularly aggressive MS that was on track to disable Molson entirely — and potentially kill her — is now virtually eliminated from her body.
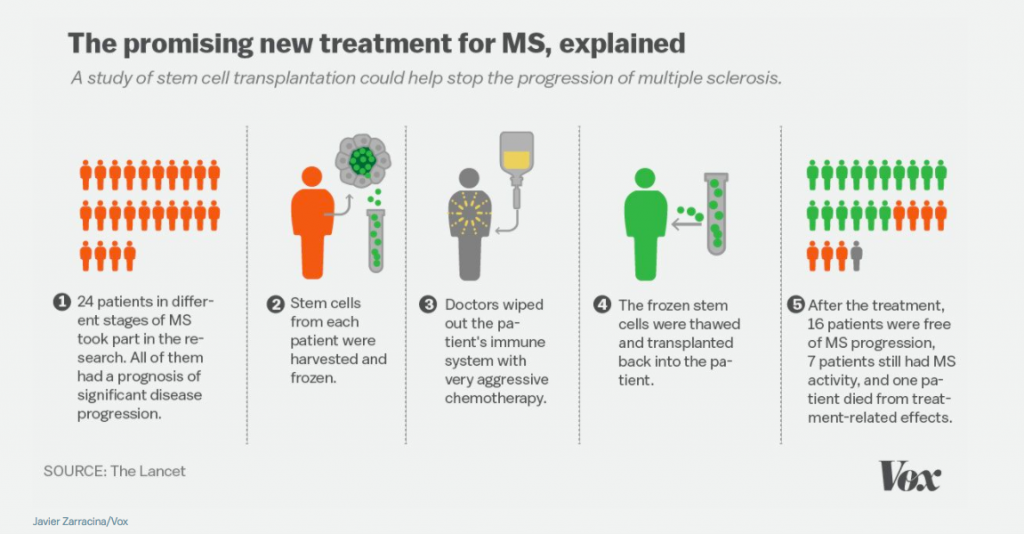
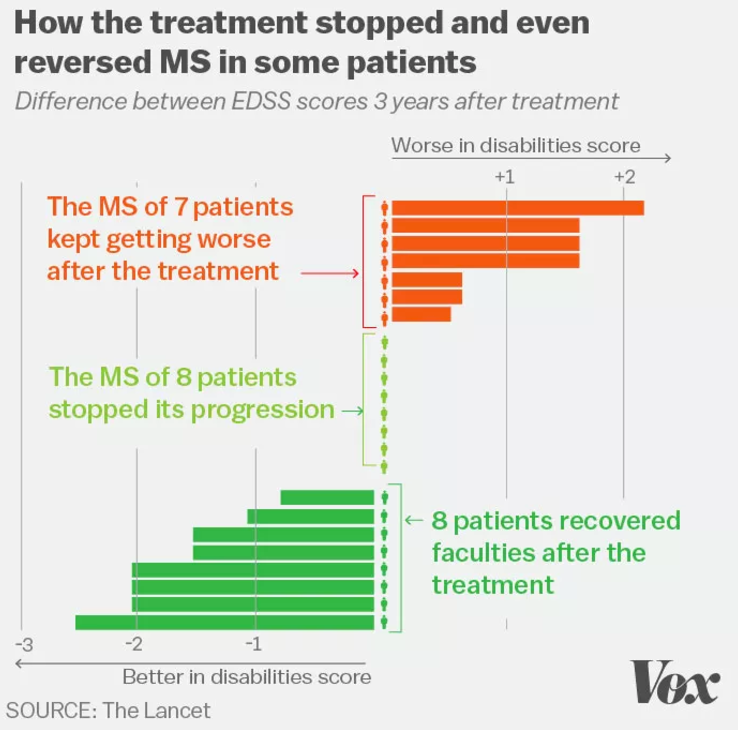
The game changer for Molson was an experimental chemotherapy and stem cell bone marrow transplant she got in 2002 as part of a study in Canada. Molson was one of a small cohort of 24 people with MS who received the high-risk therapy. Of the 24 patients, 70 percent saw the progression of their disease halted or reversed.
Their experience, documented in a paper published today in the Lancet, is the first to describe any MS treatment that fully stops the disease over the long term without MS medication.
“Everyone is hesitating to use the ‘c word,’ but these patients are cured,” says Michael Rudnicki, director of the Regenerative Medicine Program and the Sprott Centre for Stem Cell Research at the Ottawa Health Research Institute, who was not involved with the research. “Jennifer Molson was in a wheelchair in a rehab center unable to work. And now she’s skiing, she’s working, she got married, got her driver’s license. I think this is going to be the new standard of care for progressive MS.”
“My greatest hope was that the disease would stabilize”
MS affects about 2.5 million people around the world, particularly women in more temperate climates like Canada and the northern US.
Instead of protecting the body from foreign invaders, in patients with MS the immune system turns on its host, wreaking particular damage on the myelin, a protective coating around the nerve fibers in the brain and spinal cord.
Eventually these attacks can severely damage and destroy the nerves and myelin, interrupting the communication between the brain and body and leading to symptoms like numbness, trouble walking, and even blindness.
But not all patients’ symptoms manifest in the same way. People with “relapsing remitting” disease experience MS in fits and starts — their symptoms show up for a few days or weeks, followed by weeks, months, or even years of remission.
For most patients with this version of the disease, those periods of remission get smaller over time and eventually disappear, moving them into a new phase of disease known as “secondary progressive MS.”
 Javier Zarracina/Vox
Javier Zarracina/Vox
Other patients experience something even more insidious and grinding. It’s called “primary progressive MS,” and instead of bouts of remission they see a continuous increase in symptoms accompanied by a decline in health.
Molson had secondary progressive MS at the time of her treatment in 2002. She had tried medication, and nothing worked.
Her doctor, Mark Freedman (one of the authors on the Lancet paper), told her the stem cell transplant wasn’t going to cure her, but at best, if the procedure went well, her health wouldn’t worsen.
“My greatest hope was that the disease would stabilize,” Molson says. “I looked at Aaron, [now] my husband, and said, ‘If I don’t do this, and it proves to work, I’d be kicking myself if my disease progressed.’ It felt like I really didn’t have a choice.”
How stem cells gave Molson a new immune system
Once she was accepted into the study, doctors began the procedure. They first put her through a short course of chemotherapy to stimulate the production of hematopoietic stem cells, which regenerate the immune system, in her blood. They then hooked her up to a machine that cycled through her blood 32 times over the course of seven hours, in order to collect stem cells. Those stem cells were then purified, wiped of any memory of the disease, to later be transplanted into Molson through a blood transfusion.
The most trying part of the treatment: Molson had to endure 10 days of chemotherapy. The doctors were essentially killing off her diseased immune system, only to later replace it with a new one in the form of her own purified stem cells. But the experience was grueling. Molson likened it to “hell.”
“I had a feeding tube because I was constantly nauseous,” she says. “I couldn’t keep my food down.”
Growing a new, disease-free immune system meant Molson’s body had to relearn how to defend itself from disease-causing bacteria and viruses. She had to get her childhood vaccines again. But she was also more vulnerable to infection, and wound up developing a blood infection, shingles, and serious bladder infections shortly after the transplant.
/cdn0.vox-cdn.com/uploads/chorus_asset/file/6625021/MS_treatment1.jpg)
“It took me at least a year before I started to feel human again,” Molson says.
Around a year and half after the transplant, she began to slowly return to some likeness of her pre-MS self: She was walking without using a cane, she could shop in the grocery store again, and eventually she didn’t need to nap every afternoon.
“I thought, Maybe I can go back to work,” she says. By 2006, four years after her treatment, she returned to work full time. (She chose to work at the Ottawa Hospital, she said, after seeing firsthand how research can change people’s lives.)
Molson, now 41, says, “I haven’t had any MS symptoms in 14 years. And I’m not on any MS medications.” That’s a big deal given that the medications usually involve daily pills or frequent injections.
But treatment has also left her several unpleasant side effects. Because the chemotherapy damaged her ovaries and put her into early menopause, she’s on hormone replacement therapy — and wouldn’t be able to get pregnant. While she was given the chance to harvest her eggs, she declined. “I couldn’t look after myself, how could I raise children?,” she says. “Remember, I wasn’t supposed to get better.”
She also has to be extra cautious about any exposure to infections. “I was at the dentist three weeks ago, and I had to be on antibiotics before I went for a teeth cleaning,” she says. “There are still parts of the immune system that you have to be careful about.”
Molson’s hair never fully grew back, and she also contends with daily heartburn and digestive issues, which she takes medications for.
Otherwise, Molson says she feels as good as she did when she was 21, before her diagnosis. “I don’t take anything for granted. I got a second chance at life.”
Only 5 percent of MS patients will be eligible for this new treatment
/cdn0.vox-cdn.com/uploads/chorus_asset/file/6624945/FreedmanAtkinsCreditTheOttawaHospital.jpg)

The study that gave Molson this chance was impressive in its scope and its execution — conducted at multiple hospitals by hematologists and neurologists in Canada and lasting nearly 20 years, from conception to publication. It built on decades of basic research about MS, stem cells, and the immune system, including years of experience using the treatment in patients with blood and bone marrow cancers like leukemia and lymphoma.
Other scientists have already seen promising results in MS patients using similar treatment protocols, but none have managed what Atkins’s team has: to completely halt the disease’s attack on the brain —no relapses, no new MRI lesions in all surviving patients — and for such a long follow-up period.
Several researchers who were not involved with the experiment — and who typically are reserved about novel treatments — told Vox they were excited and hopeful for the treatment’s potential to benefit others with the disease.
“I think this is going to be the new standard of care for progressive MS,” said Rudnicki.
“It’s exciting — an important proof of principle,” said Jeffrey Gelfand, a neurologist specializing in MS at the University of California San Francisco.
Tim Caulfield, a University of Alberta professor who has been tracking stem cell research, noted the difference between this robust finding and the unfounded claims in stem cell clinics around the world: “This is a fascinating development … [and] a good example of the difference between the real clinical research and what is being marketed by the clinics providing unproven therapies — you can’t simply ‘inject’ stem cells and expect significant results.”
Still, there are major caveats to consider. This study was small and lacked a comparison or control arm. Similar, larger studies are needed to confirm the results, and it’s not clear what will happen to patients like Molson in the much longer term.
Ottawa Hospital hematologist Harry Atkins, the researcher who led the study, pointed out that only about 5 percent of MS patients would be eligible for this treatment: again, the minority who have an aggressive form of MS that’s not responding to any treatment.
/cdn0.vox-cdn.com/uploads/chorus_asset/file/6625029/MS_treatment3.jpg) Javier Zarracina/Vox
Javier Zarracina/VoxAnd not everyone in the study had results like Molson’s. Of the 24 patients, 70 percent saw the progression of their disease halt or reverse, but the other 30 percent continued to worsen.
That minority who didn’t respond, the researchers think, had MS that was already too far along by the time of their treatment. “At early stages, once the immune system quiets down, the brain can partially heal itself, so the disability tends to get better,” Atkins explained. “But as time goes on, the brain can’t repair itself.”
One patient died, which puts the potential for death from this procedure at around 4 percent, and severe infections after stem cell treatments — the kind Molson picked up, or worse — are also common.
Since there are other MS medications that are much less toxic and can help some patients, doctors only recommend considering this therapy as a last resort. (Theoretically, the treatment could be done in most blood and marrow transplant centers associated with major hospitals, Atkins said, but there are only a few that perform the procedure. Interested patients can contact the MS Society for more information.)
There’s also the cost: Atkins put it at about $50,000 to $65,000 per patient — and that’s if nothing goes wrong. (Although, MS medications now cost about as much for patients every year in the US so this therapy could actually be a money saver.)
And there are questions about the very long-term effects. It’s not clear what the next 10, 20, or 50 years look like for patients like Molson.
“We know in the long term survivors from groups that have their transplants for cancer and they’re cured and they live a long time,” Atkins said, “that they have a higher incidence of the general population of other diseases [such as heart disease and cancers].”
Already, Atkins and his team have been working on other applications for this stem cell procedure. They managed to reverse “stiff-person syndrome” — a rare neurological disease that causes the muscles in the body contract to the point of complete immobilization — in one patient. And there’s talk of applying the treatment to myasthenia gravis, another incurable autoimmune disease.
Cures in medicine are extremely rare. It’s still too early to label this treatment a cure for MS. “We have only followed our patients for up to 13 years,” Atkins said, “so it’s hard to say what the next 10 to 20 will bring. You’d want to know that before you’d call this a cure.”
For now, though, Atkins will use the word miracle. “It still appears like a miracle to me to see patients recover, and get back to the things that they were supposed to do in life,” he said. “It is very rewarding to see and it wasn’t what we expected, and we are overjoyed about it to know this treatment can help people in that way.”
Correction: An earlier version of this article misstated the worldwide prevalence of MS.
Source:


/cdn0.vox-cdn.com/uploads/chorus_asset/file/6625019/MS_treatment2.jpg) Javier Zarracina/Vox
Javier Zarracina/Vox